Regulation and function of heat stress transcription factors and molecular chaperones in protein homeostasis
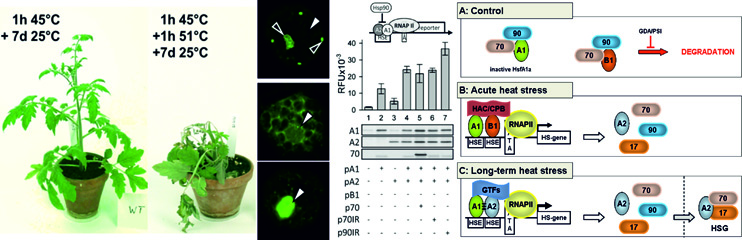
Rosenkranz RRE, Bachiri S, Vraggalas S, Keller M, Simm S, Schleiff E, Fragkostefanakis S. Front Plant Sci. 2021 Mar 29;12:645689. doi: 10.3389/fpls.2021.645689. PMID: 33854522; PMCID: PMC8039515.
Natural variation in HsfA2 pre-mRNA splicing is associated with changes in thermotolerance during tomato domestication.
Hu Y, Mesihovic A, Jiménez-Gómez JM, Röth S, Gebhardt P, Bublak D, Bovy A, Scharf KD, Schleiff E, Fragkostefanakis S. New Phytol. 2020 Feb;225(3):1297-1310. doi: 10.1111/nph.16221. Epub 2019 Nov 14. PMID: 31556121
The repressor and co-activator HsfB1 regulates the major heat stress transcription factors in tomato.
Fragkostefanakis S, Simm S, El-Shershaby A, Hu Y, Bublak D, Mesihovic A, Darm K, Mishra SK, Tschiersch B, Theres K, Scharf C, Schleiff E, Scharf KD. Plant Cell Environ. 2019 Mar;42(3):874-890. doi: 10.1111/pce.13434. Epub 2018 Oct 11. PMID: 30187931
Alternative splicing in tomato pollen in response to heat stress.
Keller M, Hu Y, Mesihovic A, Fragkostefanakis S, Schleiff E, Simm S. DNA Res. 2017 Apr 1;24(2):205-217. doi: 10.1093/dnares/dsw051. PMID: 28025318
HsfA2 Controls the Activity of Developmentally and Stress-Regulated Heat Stress Protection Mechanisms in Tomato Male Reproductive Tissues.
Fragkostefanakis S, Mesihovic A, Simm S, Paupière MJ, Hu Y, Paul P, Mishra SK, Tschiersch B, Theres K, Bovy A, Schleiff E, Scharf KD. Plant Physiol. 2016 Apr;170(4):2461-77. doi: 10.1104/pp.15.01913. Epub 2016 Feb 25. PMID: 26917685
Kontakt
Head of the Group:
Dr. Sotirios Fragkostefanakis
Biozentrum, Campus Riedberg
Gebäudeteil N200, Raum 302
Max-von-Laue-Str. 9
60438 Frankfurt am Main
T +49 69 798-29287
F +49 69 798-29286
E fragkost@bio.uni-frankfurt.de
Sprechzeiten:
Nach Vereinbarung
- Aktuelles und Presse
- Pressemitteilungen
- Öffentliche Veranstaltungen
- Uni-Publikationen
- Aktuelles Jahrbuch
- UniReport
- Forschung Frankfurt
- Aktuelle Stellenangebote
- Frankfurter Kinder-Uni
- Internationales
- Outgoings
- Erasmus / LLP
- Goethe Welcome Centre (GWC)
- Refugees / Geflüchtete
- Erasmus +
- Sprachenzentrum oder Fremdsprachen
- Goethe Research Academy for Early Career Researchers
- Forschung
- Research Support
- Forschungsprojekte, Kooperationen, Infrastruktur
- Profilbereich Molecular & Translational Medicine
- Profilbereich Structure & Dynamics of Life
- Profilbereich Space, Time & Matter
- Profilbereich Sustainability & Biodiversity
- Profilbereich Orders & Transformations
- Profilbereich Universality & Diversity





