Forschung/ Research
General research interests
Higher plants as the model plant Arabidopsis thaliana regulate their photosynthetic capacity very fast and efficient to allow for high photosynthetic performance and protection from oxidative damage at the same time. Besides photoreceptors, the photosynthetic machinery itself acts as a sensor for environmental conditions like high light, spectral changes of the incident light or drought stress. Balancing photosynthetic activity and photoprotection requires a highly sophisticated regulatory system involving the light harvesting antenna system and the photosystems themselves. Phosphorylation of LHCII and PSII core proteins induces antenna movement (state transitions) and PSII remodeling mediated by the redox-activated thylakoid kinases STN7 and STN8. Our interest is to identify PSII phosphorylation sites and understand how PSII remodeling works on molecular level in the short-term (Project 1) and in the long-term (Project 5). Further, we are interested in additional factors which are required for PSII remodeling (Project 1 and Project 3). If natural conditions are unfavorable for photosynthesis plants and algae have to protect from excessive excitation. In Project 2 we study diatom antenna complexes (fucoxanthin- chlorophyll a,c binding proteins; FCP) in vitro using liposomes with natural lipid composition. This setup allows us to mimic excessive light conditions by altering the pH and monitor spectroscopic changes in time resolved setups. Resurrection plants have the unique ability to survive drought stress and even desiccation without losing their photosynthetic apparatus. We aim to understand which genes are crucial for desiccation tolerance (Project 4).
Accomplished projects
1) “The significance of PSII supercomplexes in light acclimation”1, 3
Photosystem (PS) II supercomplexes are of substantial importance for regulation of photosynthesis. Although the structure of PSII supercomplexes has been determined, the process of PSII supercomplex assembly and PSII supercomplex remodeling during light acclimation is poorly understood. We revealed in own previous work PSII supercomplex remodeling is induced by PSII core protein phosphorylation (Dietzel et al. 2011). We aim to elucidate the assembly and regulation of PSII supercomplexes. To this end we are investigating the kinetics of PSII core phosphorylation in relation to the altered functionality of PSII supercomplexes. With fluorometrical and spectroscopical methods we assess concomitant changes in light harvesting, light energy distribution and transfer. The knowledge obtained about PSII supercomplexes and their remodeling will enable us to evaluate the effects of the light environment on plant growth and yield. In the future the productivity of agricultural important plants might benefit from this knowledge. In Collaboration with S. Baginsky (MLU Halle) and T.Pfannschmidt (Grenoble).
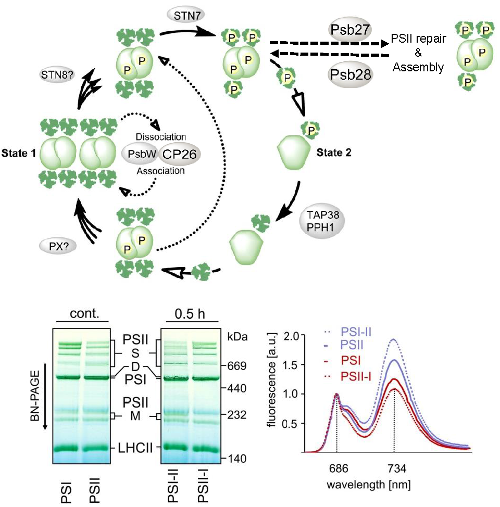 |
| PSII remodeling. Upper panel:Model for involvement of PsbW/CP26 in stabilization of PSII supercomplexes and Psb27/Psb28 in PSII assembly and repair. Lower panel: Phosphorylation dependent PSII remodeling monitored by BN-PAGE and 77 K Chl a fluorescence. |
2) “Time resolved spectroscopy of diatom light harvesting systems in proteoliposomes”2
Photosynthetic organisms such as diatoms and higher plants developed individual strategies to switch from efficient light harvesting to photoprotection. Central to this field is the so called non-photochemical quenching (NPQ) which dissipates excessive light energy as heat in a pH and xanthophyll pigment dependent manner.
Understanding NPQ on the molecular level requires detailed knowledge about the energy transfer pathways within the light harvesting systems. In this project we investigate the spectroscopic features of Cyclotella meneghiniana-FCP in light harvesting vs. photoprotective mode. To this end we use FCP reconstituted in proteoliposomes enabling us to simulate changing physiological conditions such as changes in pH and xanthophyll pigment stoichiometry. Hence, we can follow the kinetics of light energy transfer within diatom antenna systems (FCP) in close-to-nature conditions by the use of proteoliposomes. In collaboration with A. Natali, C. Ramanan, R. v. Grondelle, R. Croce (VU Amsterdam) and C. Büchel, J. Wachtveitl (GU Frankfurt)
Preliminary projects
3) “Chloroplast immunophilins in assembly and regulation of photosynthetic macromolecular complexes.”
FKBPs, initially discovered as peptidyl-prolyl isomerases (PPIase) in mammals is a protein family ubiquitous in the living world and important for cell signaling, regulation and protein trafficking. Higher plants possess the largest FKBP family which consists of 23 members in the model plant Arabidopsis and 12 of them localize to the chloroplast, more precisely, to the thylakoid lumen. Only one of them - FKBP13 - possesses PPIase activity (Gopalan et al., 2004). The second known FKBP is the redox controlled FKBP20_2 regulating the accumulation of PSII supercomplexes (Lima et al., 2006). Two more FKBPs are found to interact with PSI (Seok et al., 2014) and the NADPH-dehydrogenase complex (Peng et al., 2009). The function of the other eight chloroplast FKBPs is far from being understood. They are considered to be involved in assembly and regulation of thylakoid complexes but “how” is highly debated in the scientific community and conclusive evidence is rare.
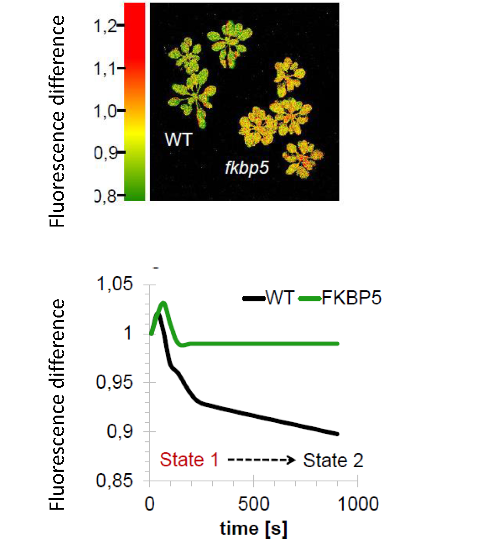 |
|
Photosynthetic phenotype of in an immunophilin mutant. Fluorcam pseudocolor image gives the difference in fluorescence between state I and II (see also Kruse 1999) indicating altered antenna size adjustment in the fkbp5 mutant. |
4) “Protection mechanisms against excess light during desiccation of the resurrection plant Haberlea rhodopensis” 4
Some plant species are able to tolerate severe desiccation. One of those, Haberlea rhodopensis, usually lives in shady habitats but lately also occurs in places exposed to full sun light. H. rhodopensis retains its chlorophyll and photosynthetic apparatus during desiccation, thus the question arises how these pigmented proteins are protected in full sunlight from photodamage under severe water-stress, when no photosynthesis can take place.
To this end we compared shady grown H. rhodopensis and plants grown in full sunlight in their natural environment concerning protection mechanism and proteins involved in desiccation tolerance, during desiccation as well as during recovery. In collaboration with K. Georgieva (Bulgarian Academy of Sciences, Sofia) and C. Büchel.
5) “Redox signaling during light quality acclimation” 5
Changes in quality induce changes in the reduction/oxidation (redox) state of the photosynthetic electron chain such as the mobile electron carrier plastoquinone (PQ) that acts as a trigger for compensatory acclimation responses like redox-controlled changes in plant gene expression in the nucleus and organelles. We identified early redox-regulated genes (ERGs) in Arabidopsis thaliana responding significantly 30 or 60 min after the generation of a PQ-reduction signal. The results reveal a significant impact of chloroplast redox signals on genes coding for the mitochondrial electron transport chain, tetrapyrrole biosynthesis, carbohydrate metabolism, and signaling lipid synthesis. These groups of ERGs are unique to redox imbalances in photosynthetic electron transport and were then used for analyzing potential redox-responsive cis-elements, trans-factors, and chromosomal regulatory hot spots. The data identify a novel redox-responsive element and indicate extensive redox control at transcriptional and chromosomal levels that point to an unprecedented impact of redox signals on epigenetic processes.
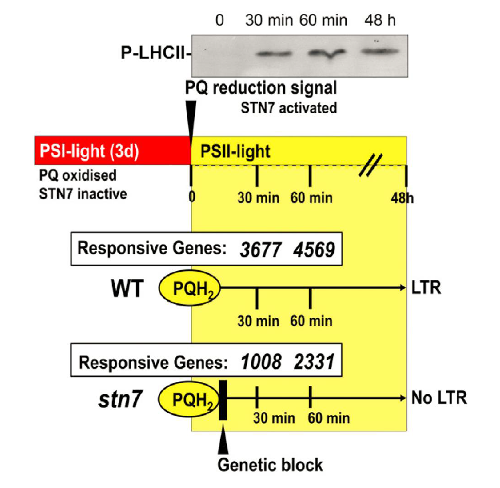 |
| Redox-controlled changes in gene expression. PQ was reduced by shifting from PSI favoring light to PSII favoring light. PQ-reduction leads to activation of the LHCII-Kinase STN7 which activates a (still) unknown signal transduction cascade towards the nucleus inducing the long-term response to light quality. In the stn7 KO the response is blocked (Bonardi et al. Nature 2005). Figure from Dietzel et al. 2015. |
In collaboration with B. Grimm and T. Börner (HU Berlin) and T. Pfannschmidt (Grenoble) and K. Mayer (Helmholtz Munich)
1DFG funded DI 1956/1-1; 2Feodor-Lynen-Fellowship of the Humboldt Foundation; 3 Young Researchers in FOKUS starting grant of the Goethe University; 4 co-applicant in a DAAD grant to C. Büchel and K. Georgieva; 5 DFG research unit 804 “Retrograde signalling in plants” to T. Pfannschmidt
href="https://www.jove.com/video/58017/isolating-incorporating-light-harvesting-antennas-from-diatom" Back to project 2.
Kontakt
Dr. Lars Dietzel
Biozentrum, Campus Riedberg
Gebäudeteil N100, Raum 0.12
Max-von-Laue-Str. 9
60438 Frankfurt am Main
T +49 69 798-29562
E dietzel@bio.uni-frankfurt.de
Sprechzeit
nach Vereinbarung
- Aktuelles und Presse
- Pressemitteilungen
- Öffentliche Veranstaltungen
- Uni-Publikationen
- Aktuelles Jahrbuch
- UniReport
- Forschung Frankfurt
- Aktuelle Stellenangebote
- Frankfurter Kinder-Uni
- Internationales
- Outgoings
- Erasmus / LLP
- Goethe Welcome Centre (GWC)
- Refugees / Geflüchtete
- Erasmus +
- Sprachenzentrum oder Fremdsprachen
- Goethe Research Academy for Early Career Researchers
- Forschung
- Research Support
- Forschungsprojekte, Kooperationen, Infrastruktur
- Profilbereich Molecular & Translational Medicine
- Profilbereich Structure & Dynamics of Life
- Profilbereich Space, Time & Matter
- Profilbereich Sustainability & Biodiversity
- Profilbereich Orders & Transformations
- Profilbereich Universality & Diversity





