The Fish Embryo Toxicity Test, the Daphnia Immobilization Test and the Algae Growth Inhibition Test - also called the aquatic trias - are among the fundamental assays performed in the Students' Lab: Goethe Goes Environment. Students and research fellows will provide a brief overview of each assay in the following sections.
Fish Embryo Toxicity Test (FET)
One of the primary goals here at E3T is to follow the recommendations of the 3-R's concept in animal testing. The 3-R's principle includes (1) Reduction of the number of animals used, (2) Refinement of the assay procedure, and ultimately, (3) Replacement of animal testing with new alternative methods. The Fish Embryo Toxicity Test (FET) is a bioassay designed as an alternative to the original acute fish toxicity test. The FET is utilized to assess the acute toxicity of environmental samples on embryonic stages of fish (teratogenicity). Emerged as a novel tool in ecotoxicological research, the FET is a well-established assay, which has been validated in multiple international guidelines (DIN EN ISO 15088: 2009-06 and OECD Test No. 236).
The FET is conducted with embryos of the zebrafish (Danio rerio), an outstanding model organism in ecotoxicology studies. Zebrafish are easy to maintain and can reproduce all year. One clutch can contain up to 200-300 eggs, and the regeneration time of the female fish is relatively short. The main advantages, however, are the transparent chorion, which enables a simple examination of the embryo, the fast embryo development, and the high sensitivity to environmental pollutants.
The FET is conducted in small glass dishes, however, a miniaturized version using 96-well plates was developed and is part of our current assay-portfolio. Both versions examine four mortality endpoints (Figure 1), namely:
- Coagulation
- Missing heartbeat
- Lack of detachement of the tail
- Lack of somite formation (only OECD 236)
In addition to these standard endpoints we also examine additional endpoints to allow a more specific mode-of-action focused assessment of environmental samples.
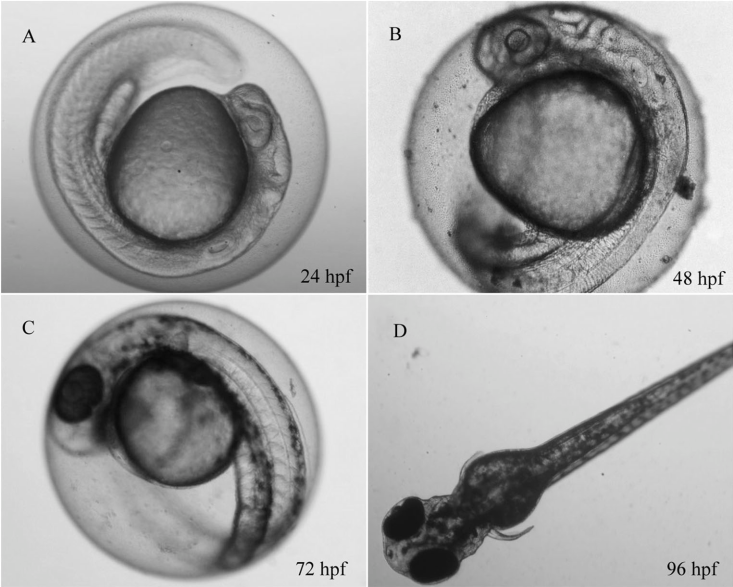
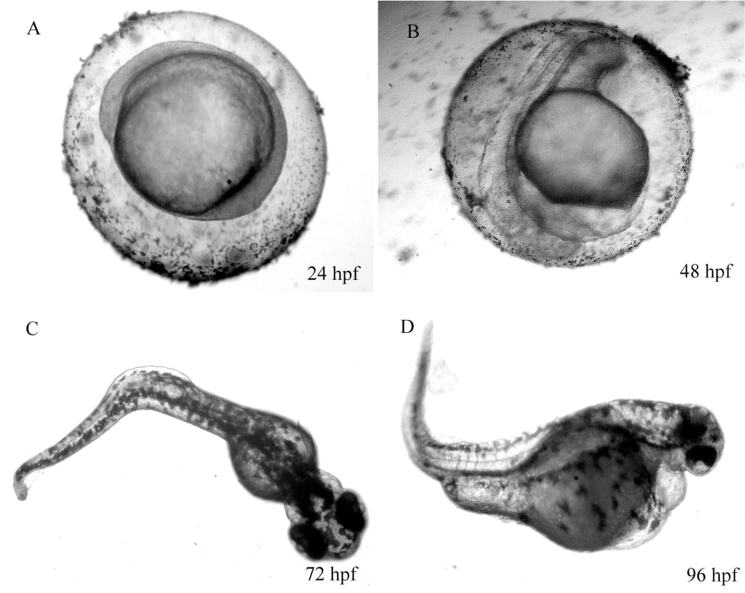
Figure 1: Danio rerio embryo development. Left: unexposed control, right: treatment. hpf: hour post fertilization
Besides the zebrafish, the freshwater Medaka (Oryzias latipes) or the Fathead Minnow (Pimephales promelas) are other common species in freshwater ecotoxicology. In contrast, established standard model organisms for marine environment research are rather scarce. In this context, our research focuses on the marine Medaka (Oryzias melastigma) as an emerging alternative laboratory model species for the brackish and marine environment. The species is endemic to the south-eastern region of Asia and can easily be cultured under laboratory conditions. O. melastigma shares some of the main advantages for embryo testing with the D. rerio, particularly a transparent chorion and a short period from fertilization to hatch (8-10 days). Further benefits regarding early life stages (ELS) testing and 'OMICs includ spawning throughout the year, a fully annotated genome, and easy sex genotyping. This combination of traits qualify O. melastigma as a model species in marine ecotoxicology, which can thus be considered a primissing marine complement to the freshwater species D. rerio. Our department is working on the further establishment of O. melastigma as a marine model species and marine animal alternative in bio testing and risk assessment. Possible applications of O melastigma may include the contect of ecotoxicological evaluation of marine oil spills or neurotoxic compounds. The marine FET with O. melastigma conducted in our lab is adapted according to standardized zebrafish procedure (DIN EN ISO 15088: 2009-06 and OECD Test No. 236).
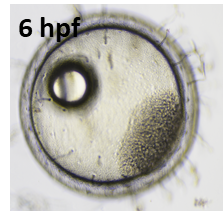

Figure 2: Medaka fertilized egg (left, 6hpf) and larvae (right, 9 dpf)
According to the EU directive 2010/63/EU the FET is considered animal-free until the time point of independent feeding. Since zebrafish and medaka embryos can start feeding shortly after hatching, we terminate the limnic and marine FET at 5 and 8 days post fertilization (dpf), respectively.
Ecotoxicity testing with the common water flea, Daphnia magna
Daphnia sp. (water flea) is a keystone species in aquatic ecosystems and belongs to the group of planktonic crustaceans living in freshwater habitats. Daphnids are an excellent model test organism in ecotoxicity testing due to their size, ease of culturing, unique parthenogenic (asexual) reproductive cycle (i.e., production of genetic clonal offspring from mothers), availability of standardized test methods, and their high sensitivity to environmental contaminants. Together with Algae and Fish, Daphnids are part of a model food chain, often referred to as the "aquatic trias" (Figure 3), and represent the primary consumers.
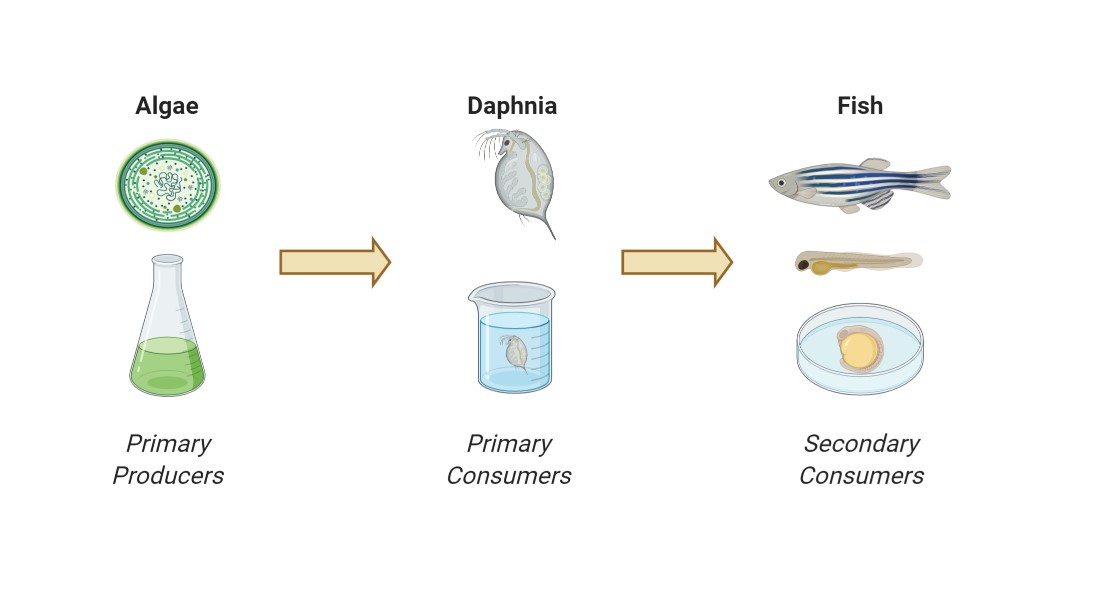
Figure 3: Ecotoxicological food chain model
By assessing ecotoxicological effects of chemicals on these three organisms, ecosystem effects regarding this simplified food chain model can be predicted. In our lab, we conduct two different types of ecotoxicological tests with Daphnia magna according to standard test guidelines:
- OECD 202 (2004) “Daphnia sp. Acute Immobilization Test" (acute 48-h)
- OECD 211 (2012) “Daphnia magna Reproduction Test" (chronic 21-d)
These tests can provide essential information about the acute and chronic effects of chemicals on these model test organisms.
The 48-h Daphnia magna immobilization test is carried out with <24 h old neonates, which aretypically exposed to control, solvent control if necessary, and exposure tretments. One assay comprises 5 individuals per replicate and 4 replicates per treatment. At the end of the test, the number of immobilized individuals is assessed. If a dose-dependent response was observed, a 48-h effect concentration of the exposure treatment causing the immobilization of 50 % of the exposed daphnids (EC50) could be determined.
In contrast, the chronic reproduction test is conducted for 21-d with <24-h old neonates. One individual per beaker is exposed to the control, solvent control, and a dilution series of the exposure treatment with 10 replicate (10 animals total) per treatment. Separation of the animals in individual beakers allows for the determination of the reproductive output over the 21-d test, in which animals are recorded daily for survival, number of living offspring produced per parental animal, determination of neonate sex, number of broods (release of neonate clutch), and length of female adults at the end of the test (Figure 4). The toxic effect of the test substance on the reproductive output is expressed as an ECx-value, calculated by fitting the data to an appropriate model using non-linear regression. Herewith, one can estimate the concentration that would cause x % reduction in reproductive output, respectively. Alternatively, the effect.threshold can be expressed as the NOEC/LOEC (No or Lowest Observed Effect Level) value.

Figure 4: Assessing the length of a female adult D. magna with eggs in the brood chamber chain
Using Daphnia to time travel:
Daphnia sp. are particularly useful in the scientific field of paleoecology as the females are cyclic parthenogens that typically reproduce asexually except under stressful conditions. Unfavorable conditions (i.e., stress) cause female daphnids to switch to sexual reproduction, and male daphnids are produced. Sexual reproduction of Daphnia sp. result in fertilized encapsulated dormant eggs (diapausing) protected by ephippium, which often sink to the bottom sediment, where tey often form a historical bank of dormant resting eggs. Burried and hidden in the sediments, these eggs can overcome centuries, waiting for better environmental conditions to hatch and help re-establish the population. They can collected via sediment cores, which help determine their approximate age, and be hatched (i.e., resurrected) under ideal conditions mimicked in the laboratory. Due to the cyclic parthenogenic reproductive cycle of the daphnids, isoclonal lineages of historical Daphnia sp. can be maintained in the laboratory for testing. The resurrected daphnids comprise two unique features: First, they historically represent the natural genetic diversity of their species in the environment; secondly, they represent the genetic difference and life-history changes that may have occurred over generations of time and evolution in which these eggs were collected and resurrected. Hence, we can display the evolutionary developments and adaptions of Daphnia sp. regarding chemical pollution and compare these to their modern-day descendants.
[Video information] Enable MediaSite contents if the message Content from MediaSite blocked appears
Growth Inhibition Test with Freshwater Algae
The Algae Growth Inhibition Test aims to provide information on the potential adverse effects of environmental samples on algal growth. Algae, which grow exponentially in controlled environments, are exposed to an unknown substance or mixture of substances for 72 hours. Treatments are compared to unexposed negative controls to determine the inhibition of the growth rate. The Algae Growth Inhibition Test may be conducted with various algal species, e.g., Pseudokirchneriella subcapitata or Desmodesmus subspicatus.
The 72-h test is carried out with algae during the phase of exponential growth. The algae are exposed in 24-well plates, and their growth rate is determined fluorometrically every 24 h.


Figure 5: Agar (left) and liquid cultures (right) of P. subcapitata
In addition to freshwater algae, we are currently establishing marine microalgae as an alternative for marine ecosystems.
Equipment used
When preparing the large amounts of exposure medium, the STARLAB ErgoOne manual pipettes were of great help. The pipettes are fast and easy to handle because of their ergonomic grip and little operating force. Due to the small size and delicate transparent structures of D. magna, zebrafish and medaka working with the Leica microscopes was particularly enjoyable. The excellent illumination of the organisms and the sharp pictures from the build-in camera made it easy to distinguish male and female Daphnia offspring and evaluating FET endpoints.

Figure 6: Evalution of the marine FET using Leica microscopes
- Aktuelles und Presse
- Pressemitteilungen
- Öffentliche Veranstaltungen
- Uni-Publikationen
- Aktuelles Jahrbuch
- UniReport
- Forschung Frankfurt
- Aktuelle Stellenangebote
- Frankfurter Kinder-Uni
- Internationales
- Outgoings
- Erasmus / LLP
- Goethe Welcome Centre (GWC)
- Refugees / Geflüchtete
- Erasmus +
- Sprachenzentrum oder Fremdsprachen
- Goethe Research Academy for Early Career Researchers
- Forschung
- Research Support
- Forschungsprojekte, Kooperationen, Infrastruktur
- Profilbereich Molecular & Translational Medicine
- Profilbereich Structure & Dynamics of Life
- Profilbereich Space, Time & Matter
- Profilbereich Sustainability & Biodiversity
- Profilbereich Orders & Transformations
- Profilbereich Universality & Diversity




